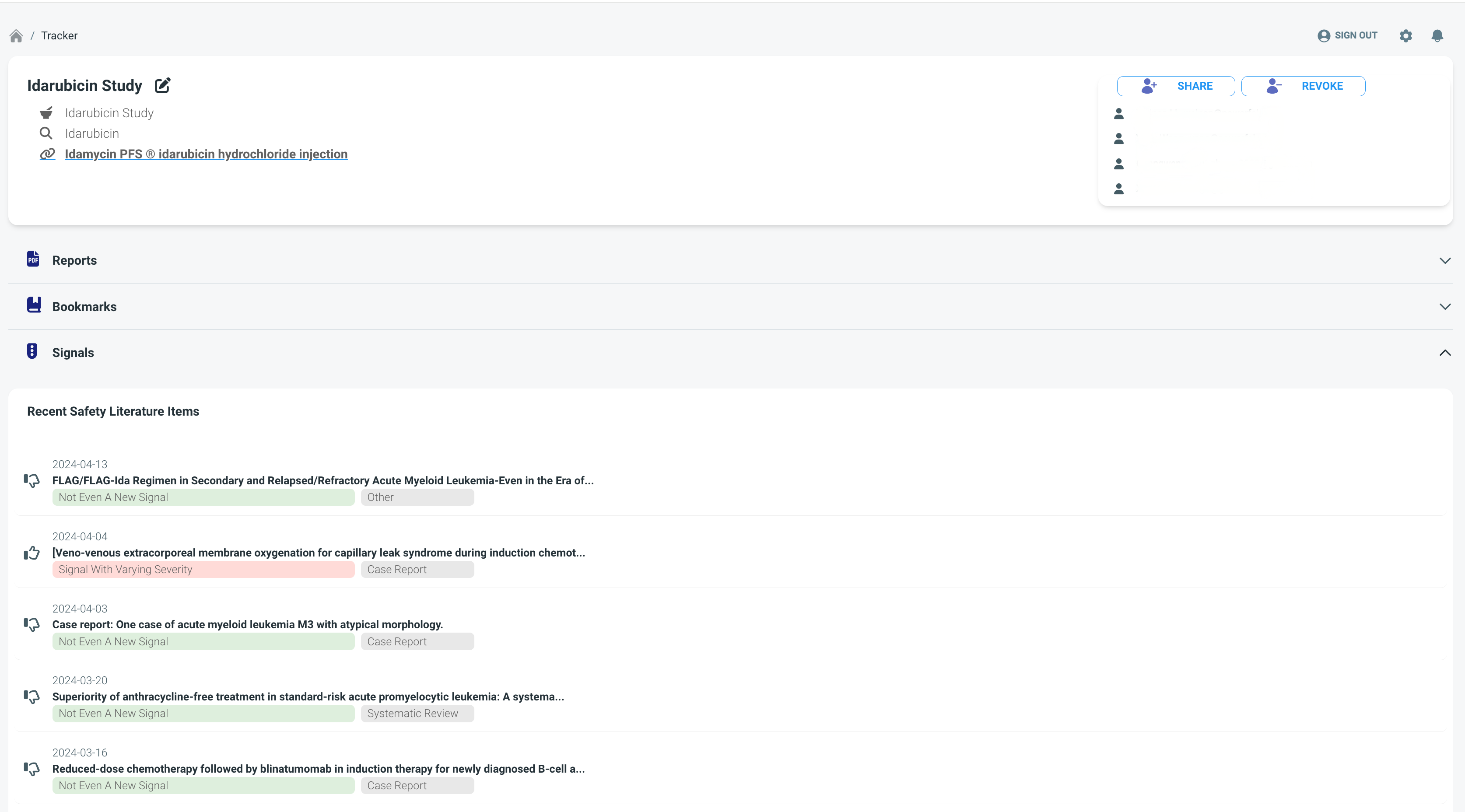
Problem
Many biopharmaceutical companies are facing challenges in tracking drug safety signals.
Solution
The PowerfAI Platform offers biopharmaceutical companies an AI-driven end-to-end workflow management software to track drug safety signals, enhancing productivity and reducing costs.
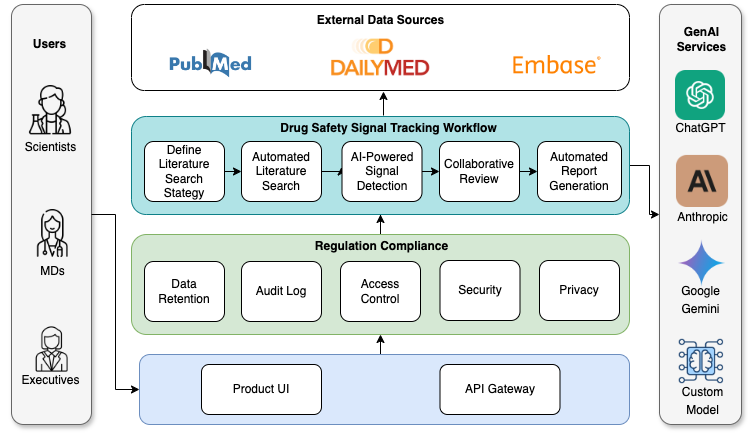
Define Literature Search Strategy
Detect potential drug safety signals and identify potential individual case safety reports (ICSR).
Automated Literature Search
Support fully-customizable search term definition, and automate literature search and article content analysis.
AI-Powered Signal Detection
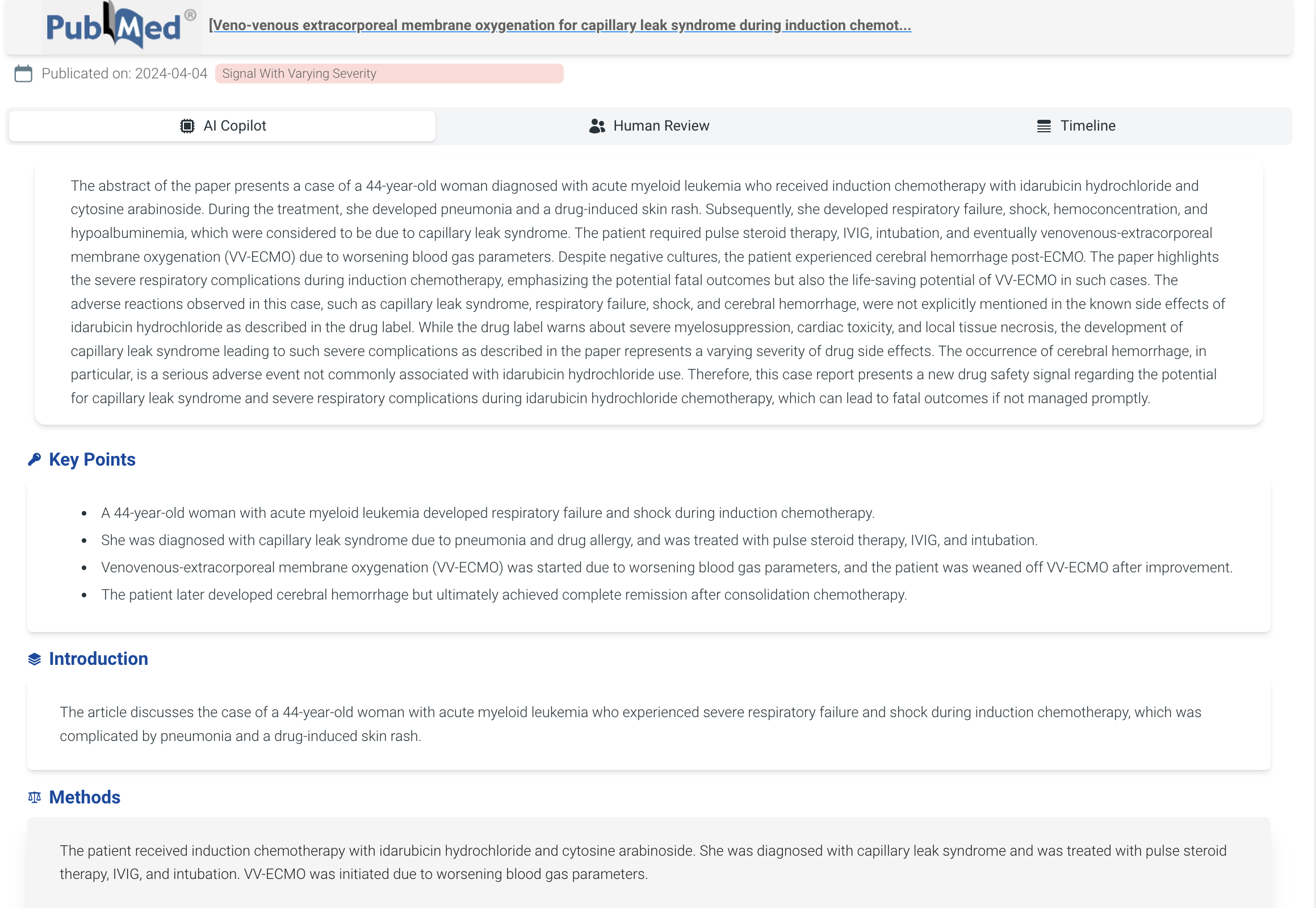
AI copilot to summarize articles with clear structured format, and detect both potential signals and adverse event case reports with detailed explanation.
Collaborative Review
Enable effective team collaboration in article review and signal decision, and preserve full working history and comments for regulation and audit needs.
Automated Report Generation
Fully customizable and user-controlled report generation through a single button click.
 PowerfAI
PowerfAI